


 email: brrob@ims.alaska.edu
email: brrob@ims.alaska.edu
 email: dkbutton@ims.alaska.edu
email: dkbutton@ims.alaska.edu
Bacterial biomass can be obtained from cell volume according to forward scatter intensity as specified by Rayleigh-Gans theory (7), cell density, and population. Values are central to formulations describing the nutrient processing ability of bacteria as well as for deriving appropriate theory on which the descriptions may be based.
Profiles of DAPI-DNA fluorescence intensity, along with population data, are used for cell cycle analysis. In combination with biomass data, information is obtained regarding cell mass accumulation during the growth cycle.
Other applications of flow cytometry in our laboratory include the identification of Hematodissidium spores (parasites) in tanner crab blood, detemination of the sex of kelp spores (6), confirmation of intact salmon brain nuclei in physiological studies of the smolting process, evaluation of the relative chlorophyll content of winter wheat chloroplasts, comparison of pigment fluorescence profiles of various tree pollens, evaluation of triploidy in salmon impinged by the Exxon Valdez oil spill, and evaluation of the effectiveness of filters used for water injection in oil wells on the North Slope of Alaska.
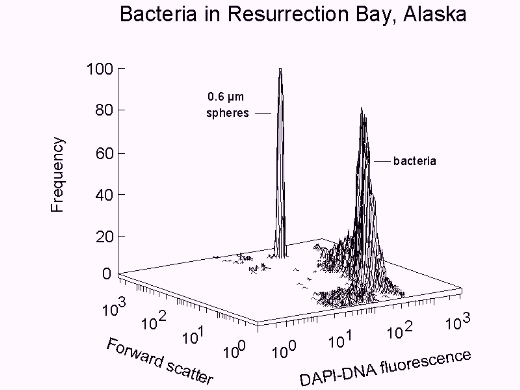
Fig. 1. Bacteria in Resurrection Bay, Alaska, stained with DAPI. Population: 6.1 x 105/ml; mean cell volume, from Rayleigh-Gans theory: 0.107 µm3; apparent DNA content: 2.3 fg/cell.
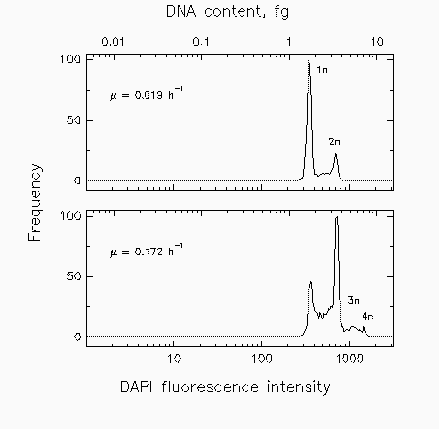
Fig. 2. Distributions of marine isolate Cycloclasticus oligotrophus grown at two different rates (µ) under acetate-limiting conditions for cell cycle analysis.
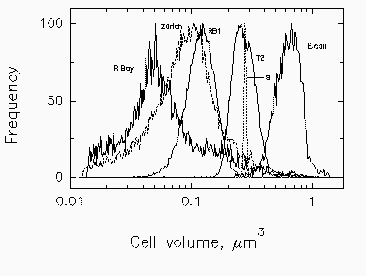
Fig. 3. Mean cell volume, computed from forward scatter intensity according to Rayleigh-Gans theory, with corrections for relative refractive index of Cycloclasticus oligotrophus RB1, Marinobacter sp. strain T2, and E. coli. Profiles for bacteria in Resurrection Bay, Alaska, and in Lake Zurich, Switzerland, are based on data for marine isolate RB1. The volume indicated for the narrow profile of standard 0.6-µm microspheres (s) is overestimated due to the high relative refractive index of polylatex.
2. Button, D. K., and B. R. Robertson. 1993. Use of high-resolution flow cytometry to determine the activity and distribution of aquatic bacteria, p.163-173. In P. F. Kemp, B. F. Sherr, E. B. Scherr, and J. J. Cole (eds.), Handbook of methods in aquatic microbial ecology. Lewis Publishers, Ann Arbor, Michigan.
3. Button, D. K., B. R. Robertson, and F. Juttner. 1996. Microflora of a subalpine lake: bacterial populations, size, and DNA distributions, and their dependence on phosphate. FEMS Microbiol. Ecol.
4. Button, D. K., B. R. Robertson, D. McIntosh, and F. Juttner. 1992. Interactions between marine bacteria and dissolved-phase and beached hydrocarbons after the Exxon Valdez oil spill. Appl. Environ. Microbiol. 58:243-251.
5. Button, D. K., F. Schut, P. Quang, R. Martin, and B. R. Robertson. 1993. Viability and isolation of marine bacteria by dilution culture: Theory, procedures and initial results. Appl. Environ. Microbiol. 59:881-891.
6. Druehl, L. D., B. R. Robertson, and D. K. Button. 1989. Characterizing and sexing laminarialean meiospores by flow cytometry. Mar. Biol. 101:451-456.
7. Koch, A. L., B. R. Robertson, and D. K. Button. 1996. Deduction of the cell volume and mass from forward scatter intensity of bacteria analyzed by flow cytometry. J. Microb. Met. In press.
8. Robertson, B. R., and D. K. Button. 1989. Characterizing aquatic bacteria according to population, cell size and apparent DNA content by flow cytometry. Cytometry 10:70-76.
 Back
to Flow Cytometry and Microbiology Introductory Page
Back
to Flow Cytometry and Microbiology Introductory Page
