


ANTIMICROBIAL SUSCEPTIBILITY TESTING USING FLOW CYTOMETRY
Kuo-Ping Chiu, Diana Davis and Craig Hixson
Bio-Rad Laboratories
Molecular Diagnostics Division, Flow Cytometry Department
4000 Alfred Nobel Drive
Hercules, CA 94547
![]() email: Kuo-Ping_Chiu@bio-rad.com
email: Kuo-Ping_Chiu@bio-rad.com
![]() email: Diana_Davis@bio-rad.com
email: Diana_Davis@bio-rad.com
![]() email: Craig_Hixson@bio-rad.com
email: Craig_Hixson@bio-rad.com
INTRODUCTION
Recent reports have illustrated that flow cytometry may be utilized
to rapidly detect antibiotic susceptibility of bacteria. This technology
offers considerable advantages in drug research for the detection of novel
antimicrobials and may, with further development, find a use in the clinic
for more efficient susceptibility testing as an aid to the selection of
therapy. The Bio-Rad Laboratories Flow Cytometric Antimicrobial Susceptibility
Test (FASTTM-2 kit) does not rely on growth inhibition, but
on the rapid detection of antimicrobial effect on bacteria measured by
a pair of fluorescent probes. Two models for antibiotic activity, Escherichia
coli and ampicillin, and Enterococcus faecium and vancomycin
were studied as a function of dosage and exposure time respectively. Transitions
in light scatter patterns of the bacteria were observed following treatment
with antibiotic at various drug concentrations and exposure times. As the
antibiotic concentration and exposure times were increased, a reduction
in the number and viability of the bacteria, as measured by the Bio-Rad
BRYTE HSTM; flow cytometer and the FAST-2 kit, was observed.
These experiments defined the effectiveness of ampicillin against E.
coli as a function of its concentration and E. faecium and vancomycin
as a function of exposure time. The results indicate that the FAST-2 system
is a rapid, reproducible and sensitive technique to study the mechanisms
of antibiotic action in bacteria.
MATERIALS AND METHODS
Reagents and solutions. Ampicillin and vancomycin were obtained from Sigma Chemical Company (St. Louis, MO) and were suspended in sterile double distilled water. The fluorescent stains (Reagent A and Reagent B) were those found in the commercially available product, Flow Antimicrobial Susceptibility Test Kit # 2 (FASTTM -2 Kit), from Bio-Rad Laboratories (Hercules, CA). LB Luria-Bertani (LB) and Brain-Heart Infusion (BHI) liquid media were obtained from Difco Laboratories (Detroit, MI).
Bacterial cultures. Escherichia coli 25922 (E. coli) and Entercoccus faecium 19434 (E. faecium) were obtained from ATCC (Rockville, Maryland). Bacteria were cultured on solid medium plates or in broth. The number of CFU (colony-forming units), an indicator of viable bacterial numbers, was used as an independent estimate of viability for all antibiotic susceptibility experiments. The CFU number was determined on plates using standard techniques.
Antibiotic Susceptibility Testing. Bacteria were inoculated into liquid medium and cultured until O.D.600 nm was approximately 0.2 (~3 hours). The concentration of ampicillin was varied from 0 to 40 µg/ml and the exposure time was fixed at 30 minutes using the E. coli-ampicillin model. Vancomycin concentration was fixed at 1.5 µg/ml and exposure times were varied from 0 to 3 hours using the E. faecium-vancomycin model. An untreated culture was included with each experiment as a control.
Staining of Bacteria with FAST-2 kit. Following treatment with antibiotic, cultures were analyzed for morphology and viability using the FAST-2 kit according to the protocol. Tubes containing no dye and each dye alone were included to set proper fluorescence compensation. A tube containing NaCl and bacteria was also included to set autofluorescence levels.
Flow cytometry. Flow cytometric analysis was carried out using a Bio-Rad Laboratories BRYTE HSTM flow cytometer equipped with a high-pressure 75 W Xenon arc lamp (Hamamatsu) and a 200 µm nozzle. Excitation light was selected using the dual exciter Bio-Rad FITC-520 optics block (470-490 nm and >520 nm excitation light). Green and red emitted fluorescence were detected using a Bio-Rad GR1 separator filter block (515 - 565 nm and 590 - 720 nm emission light). Both fluorescence emission signals were compensated to adjust for overlapping emission signals from the two fluorophores. The data were subsequently displayed and analyzed using WindowsTM-based WinBryteTM software (Bio-Rad).
RESULTS
In the first model studied, E. coli were exposed to various
concentrations of antibiotic for 30 minutes and analyzed using the FAST-2
kit and the BRYTE HS flow cytometer. Bacteria exposed to ampicillin concentrations
up to 10 µg/ml exhibited an increase in size and granularity as is
shown by a shift up and to the right in the light scatter histogram as
compared to the position of the original population (Figures
1B through 1D). This phenomenon reflects the mode of action of ampicillin,
which is to inhibit cell wall synthesis and cause filamentation, or enlargement
of the bacteria, and has been previously reported (1,2). At 40 µg/ml,
the bacteria began to lyse and a substantial amount of subcellular debris
was visible below and to the left of the intact bacteria.
Figures 2A-D show ampicillin-treated E. coli stained with the FAST-2 kit. Live, untreated E. coli stain predominantly with Reagent B and are located in quadrant A (94.7%)(Figure 2A). Ampicillin-treated E. coli exhibited four populations: a quadrant A population, positive for Reagent B, negative for Reagent A, assumed to be live cells, a quadrant B population, positive for both Reagent A and Reagent B, assumed to be dying cells, and a quadrant D population, negative for Reagent B, positive for Reagent A, assumed to be dead, intact cells. Finally, quadrant C events, negative for both stains, were cells which did not exhibit fluorescence above background levels. As the ampicillin concentration was increased, the percentage of dead and dying cells and debris also increased. At 1.25 µg/ml of ampicillin, a significant amount of cell death occurred (38.5%). The maximum effect of ampicillin was observed at 40 µg/ml (98.0%), although at 10 µg/ml, 92.6% of the cells were dead.
In the second model studied, E. faecium cultures were exposed to 1.5 µg/ml vancomycin for 40 minutes, 1 hour and three hours. The bacteria were analyzed using the FAST-2 kit and the BRYTE flow cytometer and the results are shown in Figure 3. A significant amount of cell death was observed as the exposure time to vancomycin increased, with maximum cell death occurring at 3 hours.
The absolute number of live (quadrant A) cells and the CFU counts obtained by standard plating methods were compared to assess the ability of the FAST-2 system to differentiate live and dead bacteria in both experiments (data not shown). Results showed that the two methods were in very close agreement, indicating that the FAST-2 System using the Bryte HS flow cytometer is a useful, accurate method for the determination of viable cell counts.
CONCLUSION
These experiments illustrated that the effects of antibiotic action
on bacterial cells can be monitored by flow cytometry using the BRYTE HS
flow cytometer and the FAST-2 system. These data were obtained from two
model systems of E. coli and ampicillin and E. faecium and
vancomycin. These results indicate the power of this method to study drug
concentration and kinetic effects. Flow cytometry allows for the rapid
measurement of subtle dose-response effects and has the potential to supersede
conventional growth-inhibition techniques in terms of speed, convenience,
reproducibility, and analytical performance.
REFERENCES
Gant, V.A., Warnes, G., Phillips, I. and Savidge, G.F. 1993.
The application of flow cytometry to the study of bacterial response to
antibiotics. J. Med. Microbiol. 39, 147-154.
Walberg, M., Gaustad, P., and Steen, H.B. 1996. Rapid flow cytometric
assessment of mecillinam and ampicillin bacterial susceptibility. J. Antimicrobial
Chemotherapy. 37, 1063-1075.
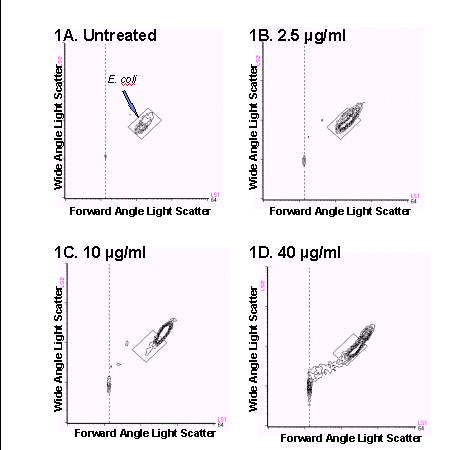
Figure 1. Bivariate light scatter cytograms obtained after treatment
of E.coli with (A) 0 mg/mL,
(B) 2.5 mg/mL, (C) 10 mg/mL, and (D) 40 mg/mL ampicillin. A reference
box was drawn
around the cells to illustrate the changes in bacterial size and granularity
following treatment with ampicillin.
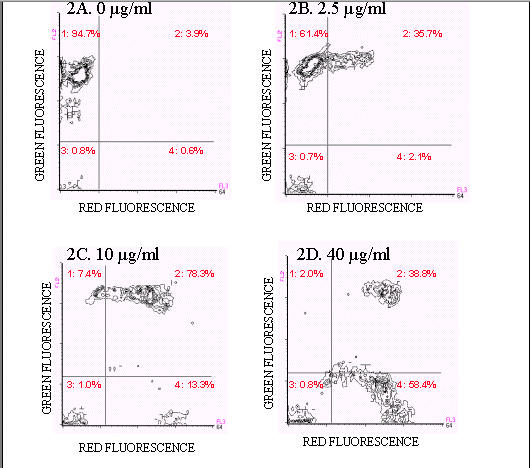
Figure 2. Two color fluorescence histograms of E.coli
treated with ampicillin and stained with
the FAST-2 kit. E.coli treated with ampicillin showed
an increase in the number of dead and
dying cells (quadrants 2, 3, and 4) and a decrease in the number of
live (quadrant1) cells (Figures 2B-2D).
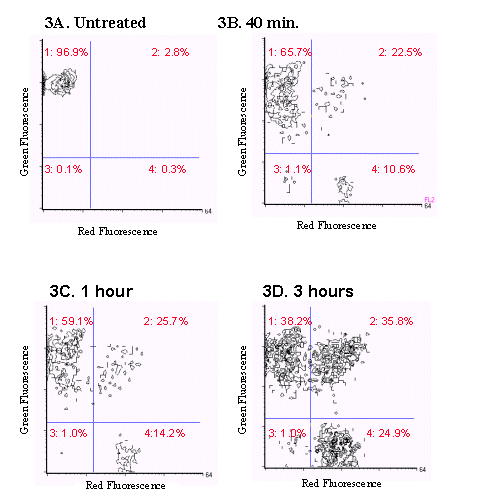
Figure 3. Two color fluorescence histograms of E.faecium
treated with vancomycin and stained
with the FAST-2 kit. As the exposure time of E.faecium
to vancomycin increased, an increase
in the number of dead and dying cells (events present in quadrants
2,3, and 4) was also observed.
 Back
to Flow Cytometry and Microbiology Introductory Page
Back
to Flow Cytometry and Microbiology Introductory Page
