Antibody Calibration
(curves: laboratories #1-9)
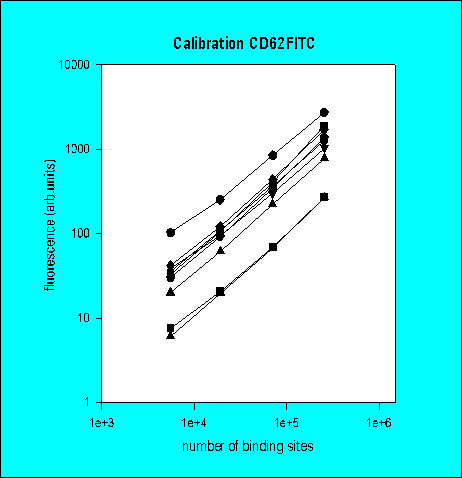
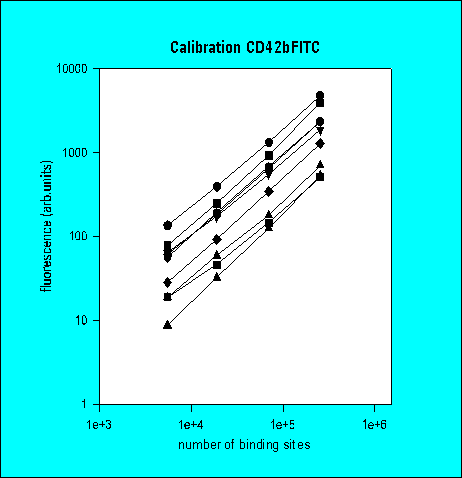
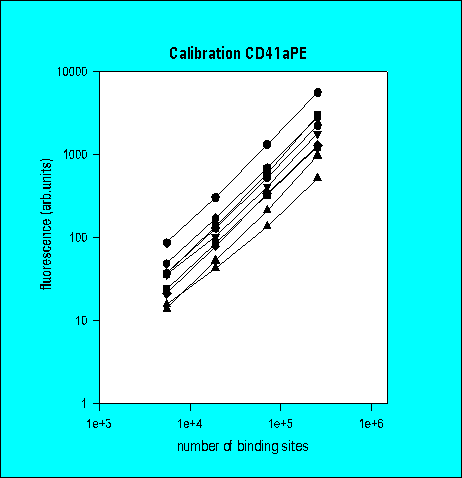
Total Antibody Binding
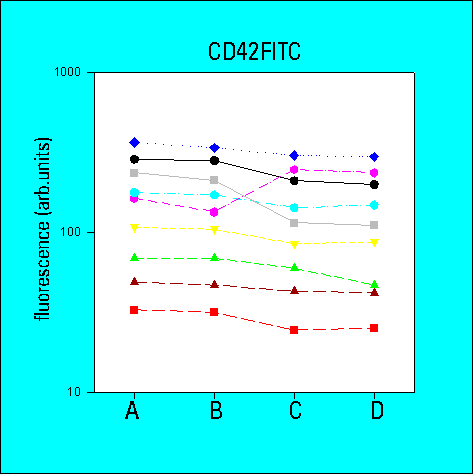
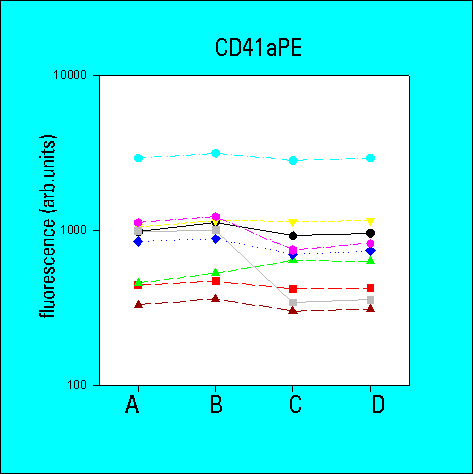
Number of Antibody Binding Sites
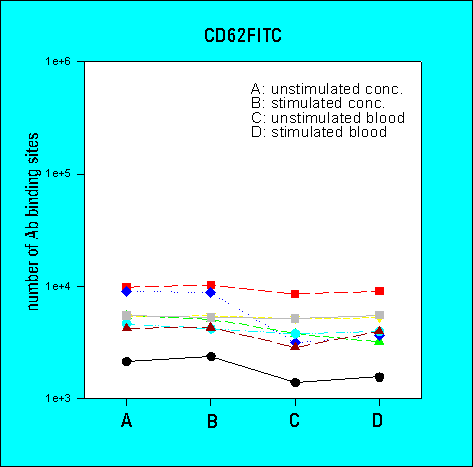
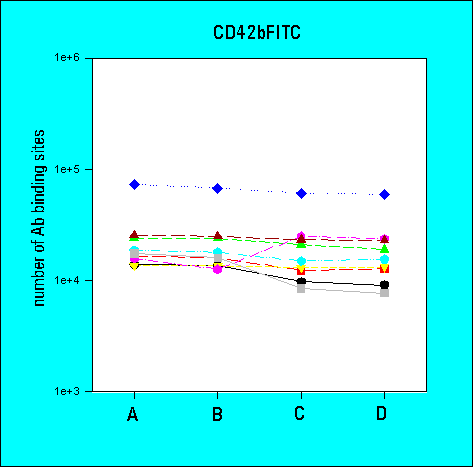
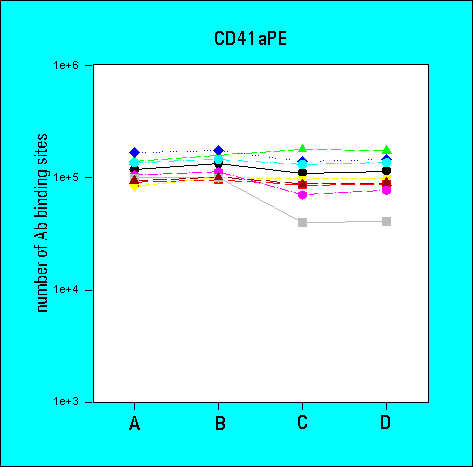
3rd Ring Trial (1995)
Quantitative Analysis of Surface Glycoprotein Expression
AG Klin.Zytometrie, Regensburg, Germany
Participating Laboratories: 9
Problems of Clinical Thrombocyte Analysis:
The results of thrombocyte functional assays are highly dependent on:
- methodological aspects because of:
- platelet instability and artificial activation during handling and
storage
- complexity of different sample preparation techniques
- heterogeneity of platelet light scatter characteristics in whole blood
- variability of antibody clones and conjugates
- differences between flow cytometer for light scatter signals, amplifiers and
optical filters
Aims of Quality Assessment Ring Trial:
- interlaboratory comparison of quantitative surface glycoproteins analysis
using high (CD41a, CD42b) or low expression density (CD62) antigens in a
dual color assay
- interlaboratory standardization of platelet activation analysis
Samples:
- aliquots of a platelet concentrate and of whole blood,
each sample native and in-vitro activated. All samples were
stabilized by mild fixation.
- fluorescent beads with a protocol for instrument setup
- fluorescent beads with calibrated mouse antibody binding for
the calculation of antibody binding sites
- standard protocol notes for sample preparation and analysis of CD41a-PE
(GpIIb/IIIa) in combination with CD42b-FITC (GpIb) or CD62-FITC (GMP-140)
requiring the use of laboratory-specific reagents
Results:
- samples were successfully analyzed within 24h by all participants
according to the standard protocol
- list mode data revealed comparable light scatter characteristics and staining
properties of the platelets
- the determination of antibody-binding sites reduced the inter-laboratory
variability in comparison to arbitrary fluorescence intensities
- although the flow cytometric measurements were very comparable amongst
the participants, the methods of data analysis were highly variable
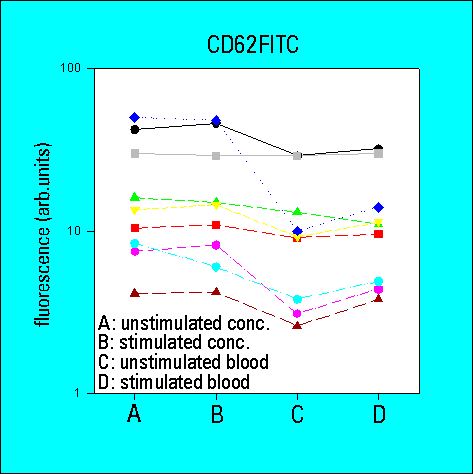
- the overall variability of results was greater for low density
CD62 than for high density CD41a or CD42b. The variability
was also higher for whole blood samples as compared to platelet
concentrates
- further standardization of the protocols will be necessary to
improve the as yet insufficient interlaboratory comparability
of the complex thrombocyte activation assay








