CONTENTS
8.1 Introduction
8.2 Specimen Collection, Transport and Integrity
8.3 Specimen Processing
8.4 Controls
8.5 Sample Analysis
8.6 Limitations of the Method
8.7 Troubleshooting
8.8 Data reporting
8.9 Quality Assurance
8.10 References
8.11 Appendix: Example of a Reticulocyte Analysis
8.1 INTRODUCTION
Reticulocytes are immature red blood cells (RBC) which have shed their nucleus, but still retain residual nuclear material. Clinically, the reticulocyte percentage is a useful indicator of erythropoiesis. In cases of anaemia, an elevated reticulocyte count is indicative of normal marrow function, whilst a decreased result may be more consistent with impaired erythropoiesis. Traditionally the reticulocyte percentage was estimated by precipitating the residual RNA with a dye, and counting the stained cells as a percentage of 1000 RBC using a microscope. This method is well known to be imprecise and open to subjective interpretation by the technologist.
Automated reticulocyte counting methods overcome most of the problems of manual counting, ie. small sample sizes and interobserver variation. The use of an automated system such as a flow cytometer for estimating reticulocyte percentages has the advantage of allowing a large number of cells to be analysed rapidly, objectively, and simply, greatly increasing accuracy and precision.
8.2 SPECIMEN COLLECTION, TRANSPORT and INTEGRITY
8.2.1 Specimen Collection
8.2.1.1 Universal precautions should be strictly observed when collecting blood samples (see 1.1 Safety Guidlines).
8.2.1.2 A RBC count should be performed on each specimen.
8.2.1.3 As EDTA is the specimen of choice for full blood estimations, it is also the most suitable specimen for reticulocyte analysis, reducing the need for a second specimen. However, any anticoagulated blood is suitable.
8.2.1.4 Specimens are suitable for analysis for up to 48 hours when stored at room temperature, or for up to 4 days at 4°C.
8.2.2 Specimen Transport
8.2.2.1 Packaging, labelling and transport of specimens should comply with all current local, state, national and international regulations for the regions through which the specimens will pass.
8.2.2.2 Specimens may be maintained at room temperature if they will be tested within 48 hours, or at 4°C if testing will be delayed.
8.2.2.3 Temperatures below 4°C or above 37°C must be avoided.
8.2.3 Specimen Integrity
8.2.3.1 Visually inspect the specimen for clots, haemolysis or container defects. Recollect the sample if the specimen shows any visual signs of deterioration.
8.2.3.2 Specimens which have been collected or transported outside of these guidelines may be processed by the laboratory according to a local approved, documented policy. The deficiencies in the sample should be noted and the report should reflect the effect that these deficiencies may have on results.
8.3 SPECIMEN PROCESSING
8.3.1 Ideally, RBC count should be performed on each specimen in order to calculate an absolute number of reticulocytes. The operator must be aware of falsely elevated reticulocyte counts where it may be necessary to perform a full blood count (FBC), or an examination of a blood film to verify the accuracy of the reticulocyte percentage (see 8.6 Limitations of the Method).
8.3.2 The flow cytometric estimation of reticulocytes is dependent upon the binding of suitable fluorescent dyes to residual erythrocyte RNA. To be suitable, a dye must have high sensitivity and specificity for RNA, should easily permeate cell membranes and give a very stable fluorescence signal. Thiazole orange gives high resolution between reticulocytes and background RBC, and is excitable at 488nm. Thiazole orange staining is stable for up to four hours at room temperature when excluded from light. Auramine O offers many of the same properties, however requires excitation at 435nm. Thiazole orange is therefore the dye of choice for use in most routine laboratories.
8.3.3 Each laboratory should establish suitable storage conditions and times for specimens prior to processing, and minimum and maximum times for incubation of stained samples, under normal operating conditions.
8.4 CONTROLS
8.4.1 Several commercially prepared assayed control materials are available for evaluating the accuracy and precision of reticulocyte analysis. Each laboratory ideally should include these with each patient run. In addition, samples which have been previously analysed can be stored at 4°C and restained and reanalysed with subsequent batches, to examine day-to-day variation.
8.4.2 The level of background fluorescence should be estimated with each batch using a small aliquot of blood added to a volume of buffered saline without the addition of the fluorescent dye. This tube is used to exclude autofluorescence and instrument noise.
8.4.3 Each laboratory should determine its own reference range (see 5.10 Determination of Reference Ranges) for reticulocytes using its particular preparation method and instrumentation, because significant laboratory-to-laboratory difference related to these variables have been reported1.
8.4.4 Instrument quality control, including laser/PMT calibration should be performed daily (see 3.0 Flow Cytometer Quality Control).
8.5 SAMPLE ANALYSIS
8.5.1 Sample order: The background and all control specimens should be run first and then, according to laboratory priority, run the patient samples. If processing large batches, controls should be run at intervals.
8.5.2 A minimum of 10,000 events per sample should be collected to allow for accurate assessment of the reticulocyte population.
8.5.3 Specimens should be acquired on LOG settings for forward and side scatter, and fluorescence parameters. The flow cytometer will detect and quantify the cells which have bound the fluorescent dye.
8.5.4 The background (unstained) sample should be run first. A gate should be set around the main population of RBC, based on forward versus side scatter, excluding platelets. A histogram of the fluorescence properties of the gated events should then be displayed, and a marker set outside the main population of unstained cells. The fluorescent events in this area represent autofluorescence and instrument noise. This background fluorescence should then be subtracted from the total fluorescence seen with the stained RBC of controls and samples to give a corrected number of reticulocytes.
8.5.5 The controls and samples should be gated using the method described above. Most of the RBC in a patient sample will not take up the dye, and appear as a large peak at the left of the fluorescence intensity plot. A marker should be set to the right of this peak, and the reticulocytes and other cells which have stained with the fluorescent dye will lie to the right of this marker (see 8.11 Appendix).
8.5.6 If an automatic program is in use, check:
8.5.6.1 Each graph to make sure you agree with the placement of the RBC gate and marker. If you consider the automatic program has selected an inappropriate position for the gate or marker, reanalyse this using an alternate program.
8.5.6.2 The appearance of the graph. If there is a large peak at 103, this may indicate a high white cell count or other cause of interference (see 8.6 Limitations of the Method).
8.5.6.7 Results should be correlated with clinical information, FBC results and film observations. Any value which is unexpected should be further investigated with reference to 8.6 Limitations of the Method.
8.6 LIMITATIONS OF THE METHOD
Since the dyes used for flow cytometric analysis of reticulocytes are nucleotide markers, there are a number of factors which, if present in a given blood specimen, may cause falsely elevated reticulocyte estimations. The following may require examination of a blood film, manual gating of the RBC population, adjustment of automatically set markers or manual estimation of the reticulocyte percentage using alternate methods:
8.6.1 Abnormally high white cell counts >20 x 109/L.
8.6.2 Patients with lymphoproliferative disorders with large numbers of small lymphocytes (8-10 microns in diameter), which will appear in the RBC gate.
8.5.3 Cases of acute leukaemia with white blood cell fragments which may be included in the RBC gate.
8.5.4 A high proportion of macrothrombocytes.
8.5.5 Howell-Jolley bodies present.
8.5.6 RBC inclusions that give rise to basophilic stippling.
8.5.7 Malarial parasites.
8.5.8 High nucleated RBC counts.
8.5.9 Unstable haemoglobins (e.g. Hb Köln which may show autofluorescence).
8.7 TROUBLESHOOTING
There are a number of causes of aberrant results which are not attributable to clinical conditions.
8.7.1 Possible causes of a reduced result:
8.7.1.1 Thiazole orange in aqueous solution is quite unstable and may deteriorate rapidly. Each laboratory should assess the stability of all working reagents under their normal operating conditions. Control results should be closely monitored as reagents may deteriorate prior to the expiry date.
8.7.1.2 The proportion of cells to reagent may be too high, quenching fluorescent tag. Prepare a fresh sample for analysis using a higher dilution.
8.7.2 Possible cause of an increased result:
Contamination - the background tube containing no stain should produce virtually no fluorescence signal. A high background may indicate contamination of the flow cytometer or the buffer solution used to prepare the staining solution, and thus false elevation of the control and patient samples. The cytometer should be cleaned in accordance with manufacturer's instructions, and a fresh background prepared in a clean tube. The run must then be repeated.
8.8 DATA REPORTING
8.8.1 Report all unique patient identifiers.
8.8.2 Reticulocytes may be reported as a percentage and/ or absolute number of the RBC population.
8.8.3 Reticulocytes should ideally be reported in conjunction with a FBC.
8.9 QUALITY ASSURANCE
Each laboratory should evaluate the quality of reagents and control specimens on a regular basis and at least when changing to new lot numbers, or preparing new solutions2. Should external quality assurance programs for reticulocytes become available, it is recommended that each laboratory participate.
8.10 REFERENCES
1. Keren DF, Hansen CA, Hurtubise PE (Ed). Flow Cytometry and Clinical Diagnosis. Chicago: ASCP Press, 1994.
2. McCarthy RC, Fetterhoff TJ. Issues in quality assurance in clinical flow cytometry. Arch Path Lab Med 1989;113: 658-666.
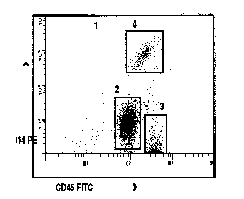
8.11 APPENDIX: EXAMPLE OF A RETICULOCYTE ANALYSIS
An example of
a reticulocyte analysis showing the RBC gate, the positioning of the marker
for estimation of the count.
