Home Cytometry History General Histories Davey

When? Important Developments in Flow Cytometry
This material was originally developed as part of a lecture given by Dr. Hazel Davey on December 1st, 2000 to third year undergraduates at the University of Wales, Aberystwyth. It his reproduced here with permission from the author. The complete, original lecture summary may be found at http://qbab.dbs.aber.ac.uk/teaching/fcmmeth.html.
In 1934 Moldovan described the first instrument that could be described as a flow cytometer, although the term flow cytometer was not coined until much later. The instrument consisted of a glass capillary tube mounted on microscope stage. Initially Moldovan used narrow tubes but found that the cells tended to block them, whilst the edges of the tubes sometimes came into the field of view and caused interference with the measurements. When wider tubes were used cells were not delivered reproducibly and errors in detection and measurement were observed.
The photoelectric counter was designed during WW2 primarily to test the efficiency of gas mask filters against particles. In the majority of experiments dust particles were used but Bacillus spores were also analysed. The device used filtered air to carry and constrain the sample rather than the sheath fluid used in modern instruments. While the air had the same effect of reproducibly delivering the sample, it obviously limited the types of biological samples that could be analysed in this device. The light source used in Gucker’s instrument was the headlight from a Ford car, yet this was sufficient to detect spores. In the conclusions to Gucker’s paper it was stated that “The principle (of flow cytometry) should have wide application in (…) bacteriology;” however, over 30 years were to pass before serious work began in microbial flow cytometry.
A device developed by Crossland-Taylor in 1953 introduced the concept of hydrodynamic focusing for reproducible delivery of cells suspended in a fluid. Using this device accurate counts of blood cells were obtained. In the same year Wallace Coulter patented the first of several devices for counting blood cells. The Coulter counter immediately superseded the Crossland-Taylor device and is still widely used today. The Coulter principle involves the detection of individual cells via electrical rather than optical means as they flow through a small orifice.
- Crosland-Taylor, PJ. A Device For Counting Small Particles Suspended In A Fluid Through A Tube. Nature 1953:171, 37-38.
- Coulter, WH. Means For Counting Particles Suspended In A Fluid. U.S. Patent #2,656,508. Application August 27, 1949. Patented October 20, 1953

In 1965 Kamentsky and colleagues described an instrument with a custom quartz flow cell which could be used for making measurements on a biological parameter. Previous systems had used flow devices only to detect and count cells. Using this device absorption of light at 254 nm was used to estimate the nucleic acid content of indiviual cells at rates of up to 500 cells/sec. Light scattering was also measured simultaneously to indicate the size of the cells with good reproducibility between samples.
Also in 1965, the Coulter principle was used in the first demonstration of cell sorting technology. The technology used to build the cell sorter came from an advance in a very different field. The deflection of electrostatically charged ink drops had recently been used in ink-jet printing and this technique was adapted to sort droplets containing cells. The Coulter volume was used to distinguish between mouse and human red blood cells (mean volumes 50 and 100 nm respectively) and then these were separated from each other with high purity. Separation of hybridoma cells from cell culture debris was also demonstrated. It was shown that the sorting process did not adversely affect the cells (viability >96% in all cases) and that the separated cells grew with an identical generation time to an untreated control.

The first commercial flow cytometer reached the marketplace in 1969. It was called the ICP 11 and was distributed by Phywe, Göttingen. A picture of this and other early flow cytometers can be seen at the Partec Flow Museum. This may be compared with a more modern flow cytometric cell sorting instrument shown in the photograph on the left. The instrument shown is a Coulter Epics Elite and was acquired by the Institute of Biological Sciences, University of Wales, Aberystwyth in August 1993. The instrument is equipped with 4 lasers and can collect data for 8 different cellular parameters. In addition it can sort selected fractions or individuals into tubes, microtitre plates or onto the surface of agar plates.
Since the 1960’s flow cytometric applications involving the study of mammalian cells have steadily increased. A variety of cell types have been analysed. Since flow cytometry requires cells to be suspended individually in fluid, blood cells are amenable to analysis with minimal pretreatment. There is growing interest in using flow cytometry routinely for the analysis of clinical specimens. Antibodies specific for different CD markers may be labelled with fluorescent tags allowing white cell typing to be carried out. This approach may be used to identify and type leukaemias or to monitor the progress of diseases such as HIV/AIDS. Other applications include analysis of cells isolated from tumors. DNA stains are used to detect aneuploidy, which is indicative of cancerous cell growth. In the research environment, flow cytometry is frequently used to study apoptosis (programmed cell death). Multiparametric flow cytometry enables researchers to monitor the biochemical changes that occur within cells as they enter an apoptotic state.
Despite the early predictions of Gucker et al. and the obvious benefits that a technique like flow cytometry has for microbial research, the first microbial flow cytometry papers did not apppear until the late 1970’s. Between 1977 and 1980 there were a few papers published which generally demonstrated that flow cytometry could be used to detect microorganisms of various types.
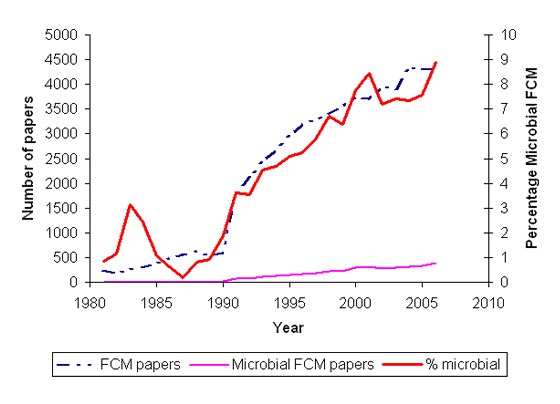
During the 1980’s Steen and coworkers demonstrated the utility of flow cytometry for studying the cell cycle of bacteria. Prior to this work, knowledge of the bacterial cell cycle was based on the work of Cooper and Helmsetter who studied division in synchronised cells in the late 1960’s. By using flow cytometry to look at individual cells, Steen and coworkers were able to use unsynchronised and thus unperturbed populations. During the last 15 years various new microbiological applications of flow cytometry have been developed and this has led to an increase in the number of microbial flow cytometry papers. The graph shown illustrates the increase in use of flow cytometry in microbiology. The graph was produced from the results of a year by year Web of Knowledge search of article titles, keywords and abstracts with the following query strings:
- flow cytometr*
- (bacteri* or microorgansim or pro?aryot* or yeast) and flow cytometr*
The value returned for the first search was used for the total flow cytometry papers published in a given year, the value for the second search was used as the microbial flow cytometry paper total. It should be noted that records prior to 1991 did not contain abstracts.
Important developments that have led to increased use of flow cytometry for microbial applications
- More powerful light sources / more sensitive detectors Microbial cells are much smaller than mammalian cells (˜1 nm rather than ˜10 nm) and consequently have lower levels of many cell constituents. For example the DNA contents of Saccharomyces cerevisiae and Escherichia coli are some 200 and 1000 times less than a normal diploid human cell. These levels were below the detection limits of the early flow cytometers. Improved optical components and design have enabled bacteria to be detected by the majority of today’s commercial instruments.
- Specialised instruments for microbiology The early work of Steen and coworkers was made possible by a purpose-built instrument that was later commercialised as the Skatron Argus 100. This instrument was much more sensitive than its contempories and therefore was ideal for measurements of bacteria. It was sufficiently sensitive for some work on larger viruses to be successfully carried out.
- Computing power In order for measurements to be made and data to be stored at rates of 100’s or 1000’s of cells per second, data acquisition cards and computers were required. Over the years the price of “sufficient power” has decreased considerably making the technique more affordable for the microbiologist. Whereas 20 years ago a “top end” computer would have been required for data acquisition, a very “run-of-the-mill” pc is acceptable today. In fact the Coulter Epics Elite in Aberystwyth is controlled from a 486DX33 using DOS-based software.
- Fluorescent stains There is a constantly growing number of fluorescent stains available for flow cytometric research. Over recent years the number of cellular targets amenable to flow cytometry has increased. This has been coupled with more stable dyes and different excitation and emission wavelengths, allowing more parameters to be measured simultaneously. While many stains designed for mammalian cell analysis can be applied to microbial research, the recent stains developed for microbial systems are undoubtedly encouraging wider application of the technique in microbiology. The online version of the Invitrogen Molecular Probes Handbook is an excellent resource for learning more about fluorescence and fluorescent stains.
- Specific fluorescently labelled probes Growth in flow cytometric research has been complemented by developments of specific probes such as antibodies and oligonucleutides. These probes can be designed with different levels of specificity allowing detection of target organisms and identification and monitoring of organisms in natural populations or laboratory consortia.
- Fluorescent reporter proteins The availability of fluorescent reporters such as GFP and its variants have allowed monitoring of gene expression in a wide variety of organisms. Using different reporters with flow cytometry has enbled expression of several genes to be monitored simultaneously in individual cells.
- Stable high-speed sorting together with sorting directly onto agar plates is of clear benefit in microbial research. This technology allows isolation of rare high producers from a mutagenised population and can be used in strain improvement programs. In addition FACS allows cells that have been provisionally identified from their flow cytometric signature to be isolated as colonies on agar plates or directly onto a microscope slide to allow confirmation of their identity.
Microbiological applications
A variety of applications of flow cytometry in microbiological research have been developed and published. These include:
- Determination of protein / lipid / DNA / RNA / product content
- Detection of target cells against particulate background
- Determination of antibiotic effects
- Determination of viability
Viability can be monitored via a variety of methods:
- Dye exclusion – e.g. PI
- Dye uptake – e.g. Rhodamine 123
- Metabolic activity – e.g. FDA
- Acetate groups cleaved from fluorescein diacetate (non-fluorescent) to liberate fluorescein (fluorescent)
- BUT fluorescein is very soluble and leaks out of cells which can lead to an underestimation of the number of viable cells
- Variants have been synthesised which become anchored in the cell
Enzyme activity
Non fluorescent substrates such as FDA are also used to indicate enzyme activity and can act as gene reporters in a similar manner to colorimetric assays involving lacZ. By using fluorescent reporters enzyme activity in individual cells can be monitored leading to a growing role for flow cytometry in microbial molecular biology. Monitoring the heterogeneity of gene expression within a population is much more powerful than studying population-based responses – particularly when one considers that cells may respond in different ways to environmental stimulii according to their position in the cell cycle.
Substrate design
It was mentioned above that fluorescein liberated from probes such as FDA tend to leak out of cells. A new compound was recently described (Zlokarnik et al. (1998): Quantitation of transcription and clonal selection of single living cells with β-lactamase as reporter. Science 279, 84-88.) which overcomes this problem.
The compound described, CCF2/AM, is a non-fluorescent precursor. It is sufficiently non-polar to easily cross the cell membrane where it is converted to fluorescent CCF2 by non-specific esterase action. CCF2 is polar and is thus trapped within the cell. β-lactamase then cleaves CCF2 into two separate fluorescent molecules with different emission and excitation wavelengths that can be independently monitored by flow cytometry.
Reporter development
An important fluorescent reporter molecule for flow cytometry is the so-called green fluorescent protein (GFP). This was originally isolated from the jellyfish Aequorea victoria (see photo at left). Production of this protein is now used as a gene reporter in both mammalian and bacterial systems. The original green protein has been modified by mutations to produce proteins which fluoresce at different wavelengths, giving a range of spectrally discrete reporters including RedFP, CyanFP, BlueFP, SapphireFP and YellowFP. This development has enabled multiparametric research into the expression of several different genes. Thus gene activity can be monitored for different genes at the same time in 1000’s of individual cells.
Conclusions
The take home message from this article may be summarised in the following two sentences:
- Flow cytometry is a very powerful technique for studying a variety of cellular characteristics at the single cell level.
- Developments in a variety of fields have advanced the field and will continue to do so.

- Dr. Hazel Davey
- Inst. Biological Sciences
- UW Aberystwyth
- Ceredigion, Wales, SY23 3DD
- hlr@aber.ac.uk
The information provided on this and other pages by me is my own personal responsibility and not that of the University of Wales, Aberystwyth. Similarly, any opinions expressed are my own and are in no way to be taken as those of U.W.A.
