Flow Cytometry and Microbiology

Rapid detection and enumeration of food-borne pathogens.
Richard Raybourne, Mary Lou Tortorello and Gabrielle Roth
FDA
MOD.1 Facility, HIS-326
8301 Muirkirk Road
Laurel, MD 20708 USA
 email: RAR@FDACF.SSW.DHHS.GOV
email: RAR@FDACF.SSW.DHHS.GOV
 email: GXR@VM.CFSAN.FDA.GOV
email: GXR@VM.CFSAN.FDA.GOV
Introduction
We are very interested in using immunofluorescent staining combined with flow cytometry as a
method to detect and enumerate food-borne pathogens. Flow cytometry offers several potential
advantages over existing rapid methods. Two of the most important are rapid time of analysis
which results in a high through put potential and the potential for automated sample analysis. Prior to the commercial availability of the Bio-Rad Bryte HS flow cytometer, microbial analysis was limited to custom built instrumentation, or to using other standard flow units
at or very near their limits of detection.
We chose the E.coli O157:H7 system because a direct epifluorescent technique had been developed for this organism in ground beef. Because the organism is dangerous even at very low levels in foods, it is common practise to use enrichment cultures to increase the number of E.coli O157:H7 relative to the background bacterial population. We conducted experiments in which enrichment cultures containing various levels of E.coli O157:H7
were analyzed by the epifluorescent technique and by flow cytometry. We found the results
to be comparable down to about 10^4 E.coli O157/mL. An unanticipated benefit of using flow
cytometry was the observation that E.coli O157 had a distinctive light scatter profile
compared to the background bacterial population. This is an example of how the
multiparameter data acquisition of flow cytometry can increase the sensitivity and resolution
of the the immunofluorescent method.
Data Examples
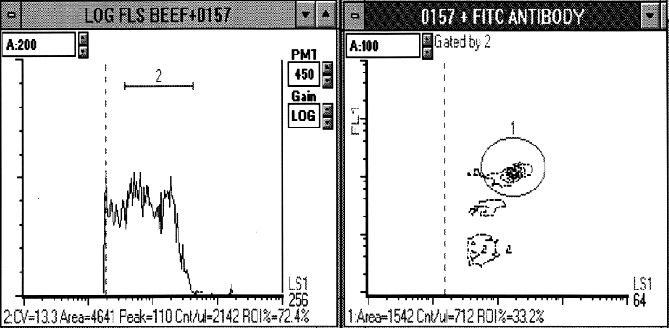
Figure 1. E.coli O157:H7 cells from a beef enrichment culture processed and stained with a
FITC anti-E.coli O157:H7 polyclonal antibody from Kirkegaard and Perry Laboratories, Inc. Enrichment cultures were used to bring low numbers of O157:H7 up to the level detectable by flow cytometry. A). Log forward light scatter (LS1) was used to define the bacterial population (region 2). B) Log forward light scatter (LS1) and log FITC fluorescence (FL1) cytogram of the gated population (region 2). The distinctive light scatter profile of the FITC positive population can be found in region 1.
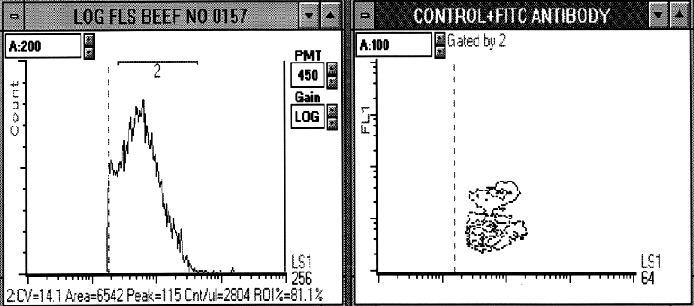
Figure 2. A negative beef enrichment culture processed and stained with FITC anti-E.coli O157:H7 polyclonal antibody. E.coli O157:H7 cells were not present in this sample. A). Log forward light scatter (LS1) was used to define the bacterial population (region 2). B) Log forward light scatter (LS1) and log FITC fluorescence (FL1) cytogram of the gated population (region 2). The intermediate staining population was not O157:H7 cells and served as a negative control.
 Back to Flow Cytometry and Microbiology Introductory Page
Back to Flow Cytometry and Microbiology Introductory Page
CD ROM Vol 2 was produced by staff at the Purdue University Cytometry Laboratories
and distributed free of charge as an educational service to the cytometry community.
If you have any comments please direct them to
Dr. J. Paul Robinson, Professor & Director, PUCL, Purdue University, West Lafayette,
IN 47907. Phone:(317) 494-0757; FAX (317) 494-0517; Web http://www.cyto.purdue.edu EMAIL robinson@flowcyt.cyto.purdue.edu




 email: RAR@FDACF.SSW.DHHS.GOV
email: RAR@FDACF.SSW.DHHS.GOV  email: GXR@VM.CFSAN.FDA.GOV
email: GXR@VM.CFSAN.FDA.GOV

 Back to Flow Cytometry and Microbiology Introductory Page
Back to Flow Cytometry and Microbiology Introductory Page