G. Mazzinia, C. Ferrarib, R. Albericia, M. Danovac and P. Dionigib
a Centro di studio per lIstochimica, Consiglio Nazionale delle Ricerche, Dipartimento di Biologia Animale; b Dipartimento di Chirurgia, Patologia Chirurgica Ia; c Dipartimento di Medicina Interna e Oncologia Medica. Università e IRCCS S. Matteo, Pavia, Italy.
DNA content per cell had been, since many decades, the most significant cellular parameter able to monitor the cell proliferation and the neoplastic transformation. Flow cytometry contributed to turn the quantitative measurement of DNA to a routine determination in clinical oncology. DNA histogram distribution of thousands of cells gives significative information on both S-phase fraction and presence of aneuploid subpopulations. More recently instrumentational and methodological improvements of flow cytometry made multiparametric analysis very powerful in biomedical applications, mainly in the area of cell proliferation studies. Other cellular parameters involved in cell growth and, perhaps in the neoplastic transformation, had been correlated to the DNA content by means of a simultaneous dual parameter flow cytometry. RNA, cellular or nuclear protein have been considered and their cytochemical quantitation had been performed simultaneously with DNA measurement. Nowadays many proliferation markers and oncogene products have been discovered and their application in clinical oncology seems to be very promising. Among others Ki-67, transferrin receptor, PCNA, ras and mic gene families are going to be extensively used for citokinetic or neoplastic characterization of tumors. By means of a monoclonal antibody immunodetection, their quantitative determination is relatively fast and simple, at least on fixed smears or tissue sections. For some of them the immunocytochemical detection on single cells and therefore the flow cytometric measurement of their level of expression is still under experimental testing. Significant improvement in cell kinetic studies have been induced by the use of Bromodeoxyuridine (BrdU), a pyrimidine analogue which is incorporated into the proliferating S-phase cells. The availability of a monoclonal antibody (MoAb) against BrdU and the possibility of a correlated analysis of BrdU/DNA contents gave to multiparametric flow cytometry a great analytical capability for tumor cell proliferation studies. BrdU can be used both "in vitro" and "in vivo" conditions. In this latter case a complete set of kinetic parameters can be acquired on a single tissue sample. Beside the BrdU labelling index (LI), other important kinetic information can be acquired including the DNA synthesis time (Ts) and the potential doubling time (Tpot) of the analysed tumor cell population.
Since 1985 we had been interested in cell proliferation studies of gastro-intestinal
tumors and few later BrdU infusion "in vivo" had been included in this
protocol. A few hours (from 3 to 6) before biopsy (or surgery) patients
are infused with 500 mg BrdU in 100 ml NaCl (under permission of the Ethical
Committee of the Department of Internal Medicine of the University of Pavia
and informed consent from each patient). DNA histogram distribution is
still the basic parameter acquired. Routine analysis includes DNA flow
cytometry (single parameter) on fresh or frozen (-20°C) samples and
BrdU/DNA (dual parameter) correlated determination on an aliquot of fixed
tissue.
Improved cell cycle kinetic analysis of solid tumors
Up to now we had been able to acquire a complete set of kinetic data and DNA index values of about three hundred patients and we can state that DNA/BrdU flow cytometry is a very important tool in tumor cell proliferation studies. The feasibility of this approach gave satisfactory results in up to 85% of the analyzed cases. Nevertheless the impact of these findings is still limited at clinical level. This may be due to the low significance of the kinetic data acquired by flow cytometry in solid tumors. In fact not only the thrue tumor cell population is analysed but also many other cell types (lymphocytes, macrophages, stromal cells, etc.) contribute to the final result. In case of epithelial tumors, cytokeratin (CK) can be used as a discriminating parameter able to esclude CK-negative cells from the flow cytometric analysis. The presence of cytokeratin filaments in the cytoplasm is a peculiarity of both normal and neoplastic transformed epithelial cells. There are many types of keratin filaments and now different monoclonal antibodies are available for many of them. In case of gastro-intestinal carcinomas or adenocarcinomas, good results (in terms of specificity) had been acquired using a MoAb specific for cytokeratins 8, 18, 19 (according to the Moll classification). Immunolabelling procedure had been verified on fixed tissue sections from either normal colon epithelial tissue and colon carcinoma. Epithelial cells had been always labelled in the cytoplasm using both directly fluoresceinated MoAb and the indirect immunofluorescent procedure. The cytoplasms of non-epithelial cells did not show any appreciable fluorescence. These findings suggested the possibility to realize an improved flow cytometric approach where the acquisition of the DNA data is controlled by the green fluorescence signal of the CK-positive cells. For this purpose whole cells are required. Single cell suspensions have to be prepared from fresh material by accurate mechanical procedures. Cell suspensions are then fixed and stored in ethanol 70%. On the BrdU treated cases we had been interested to set up a similar analytical strategy based on CK labelling. Basic requirement is the combination of three fluorochromes allowing a satisfactory separation of their fluorescence emissions. Being Propidium Iodide (PI) the fluorochrome of choise for DNA staining, FITC and phycoeritrin (PE) have been used for BrdU and CK labelling respectively (the reversed combination had also been tested). Preliminary experiments showed that the acid hydrolysis, required for the BrdU detection, didnt remove the CK filaments from the cytoplasm. Dual parameter flow cytometry allows to obtain bidimensional plots were CK-positive cells are sufficiently differentiated from non epithelial cells. Therefore the DNA analysis can be limited to the identified epithelial subpopulation, thus increasing the significance of the acquired data.
We can conclude that single parameter flow cytometry is a very powerful
tool for cell proliferation studies. Nevertheless in case of solid tumors
the significance of the data acquired, very often, cant be referred to
the real tumor cell population (because of the contamination of other cell
types). Dual parameter flow cytometry, based on the use of tissue-specific
markers, can help to solve the problem. Combination of BrdU technique "in
vivo" and three parameters flow cytometry will allow a detailed mirate
cell proliferation study of epithelial tumors.
Staining procedures
a) Single parameter DNA analysis
Treat fresh or frozen samples mechanically by means of a scalpel or by an automated tissue disaggregator ("Medimachine" Consul TS-Italy, code 79000).
Stain the obtained cell or nuclei suspension for 1 hour in 50 µg/ml PI containing 100 U/ml RNase and 0.1% Nonidet P40.
Analyze about 20,000 cells in a flow cytometer providing excitation with a 546±10 nm interference filter and selecting the red fluorescence to be measured with a 610 long pass filter.
b) Biparametric DNA/BrdU analysis
Treat whole cells or nuclei with 2N HCl for 20 min at room temperature to denature the double-stranded DNA.
Neutralize with 0.1 N Na2 B4 O7 for 5 min and permeabilize for 10 min with PBS containing 0.5% Tween-20 and 0.5% bovine serum albumine (BSA).
Incubate for 30 min with 200 µl of 1:10 anti-BrdU mouse MoAb (Becton Dickinson, Cat. N° 7583) diluted in PBS/Tween-20/BSA.
After PBS washing incubate again for 10 min with PBS/Tween-20/BSA.
Resuspend for 30 min in 200 µl of FITC-conjugated anti-mouse IgG (Techno Genetics, Cat. N° 3901-2) diluted 1:50 in PBS/Tween-20/BSA. (Indirect two step procedures have to be prefered, in flow cytometry, because of the higher signal to noise ratio obtained).
Wash twice and leave in PBS overnight.
Counterstain for 2 hours with 5 µg/ml PI, containing 100 U/ml RNase and 0.5% Nonidet P40.
Analyze with a flow cytometer performing excitation at 488 nm and collecting FL1 = green fluorescence (FITC-BrdU) at 515-540 nm and FL2 = red fluorescence (PI-DNA) above 620 nm.
c) Biparametric DNA/CK analysis
Incubate ethanol fixed cells for 30 min with 100 µl of anti-CK mouse MoAb (Becton Dickinson, Cat. N° 7650) diluted 1:20 in PBS/Tween-20/BSA.
Wash twice and incubate for 30 min with 1:50 FITC-conjugated anti-mouse IgG.
After two more washing counterstain with 5µg/ml PI containing 100 U/ml RNase and 0.5% Nonidet P40.
After 2 hours run samples in a dual parameter flow cytometer providing excitation at 488 nm and collecting fluorescences as previously described for the DNA/BrdU analysis.
d) Multiparametric DNA/CK/BrdU analysis
Treat whole ethanol fixed cells according to the BrdU protocol technique using a rat anti-BrdU MoAb (Seralab BU1/75, Cat. MA5 250) and a FITC-conjugated anti-rat IgG (Sigma, Cat. F-1763) for the second step.
Wash and incubate for 30 min with 100 µl of anti-CK mouse MoAb diluted 1:20 in PBS/Tween-20/BSA.
After washing incubate cells with 1:50 phycoeritrin (PE)-conjugated anti-mouse IgG (Sigma, Cat. P-8547).
Counterstain with PI 5 µg/ml for 2 hours.
Run in a flow cytometer able to detect three fluorescent bands: FL1 = green fluorescence (FITC-BrdU) 515-540; FL2 = orange fluorescence (PE-CK) 560-590; FL3 = red fluorescence (PI-DNA) above 620 nm; (providing adequate setting compensation due to partial overlapping of the emitted fluorescences).
References
Barlogie B, Raber MN, Schumann J, Johnson TS, Drewinko B Swartzendruber DE, Gohde W, Andreff M, Freireich EJ. Flow cytometry in clinical cancer research. Cancer Res 1983; 43: 3982-3997.
Begg AC, McNally NJ, Shrieve DC, Karcher H. A method to measure the duration of DNA synthesis and potential doubling time from a single sample. Cytometry 1985; 6: 620-625.
Begg AC, Hofland I. Cell kinetic analysis of mixed populations using three-color fluorescence flow cytometry. Cytometry 1991; 12: 445-454.
Danova M, Wilson GD, Riccardi A, Mazzini G, Ucci G, Giordano M, Brugnatelli S, Luoni R, McNally NJ, Ascari E. In vivo administration of bromodeoxyuridine and flow cytometry for cell kinetic studies in human malignancies. Haematologica 1987; 72: 115-119.
Danova M, Mazzini G, Giordano M, Ferrari C. Bromodeoxyuridine-based methods for cell kinetic studies in human tumors. Appl fluor technol 1991; 3: 1-10.
Ferreo M, Spyratos F, Le Doussal V, Desplacies A, Rouesse J. Flow cytometric analysis of DNA content and keratins by using CK7, CK8, CK18, CK19, and KL1 monoclonal antibodies in benign and malignant human breast tumors. Cytometry 1990; 11: 716-720.
Gratzner HG. Monoclonal antibody to 5-bromo and 5-iododeoxyuridine: a new reagent for detection of DNA replication. Science 1982; 218: 474-479.
Gray JW. Monoclonal antibody against bromodeoxyuridine. Cytometry 1985; 6: 499-504.
Mazzini G, Danova M, Ferrari C, Giordano M, Dionigi P, Riccardi A. Cell proliferation and ploidy of human solid tumors: methodological experience with in vivo bromodeoxyuridine and DNA flow cytometry. Anal Cell Pathol 1995; 10: 101-113.
Moll R, Franke WW, Schiller DL. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982; 31: 11-16.
Riccardi a, Danova M, Mazzini G. Bromodeoxyuridine for cell kinetic investigations in humans. Haematologica 1988; 73: 423-430.
Vindelov LL, Christensen IC. A review of techniques and results obtained in one laboratory by an integrated system of methods designed for routine clinical flow cytometric DNA analysis. Cytometry 1990; 11: 753-758.

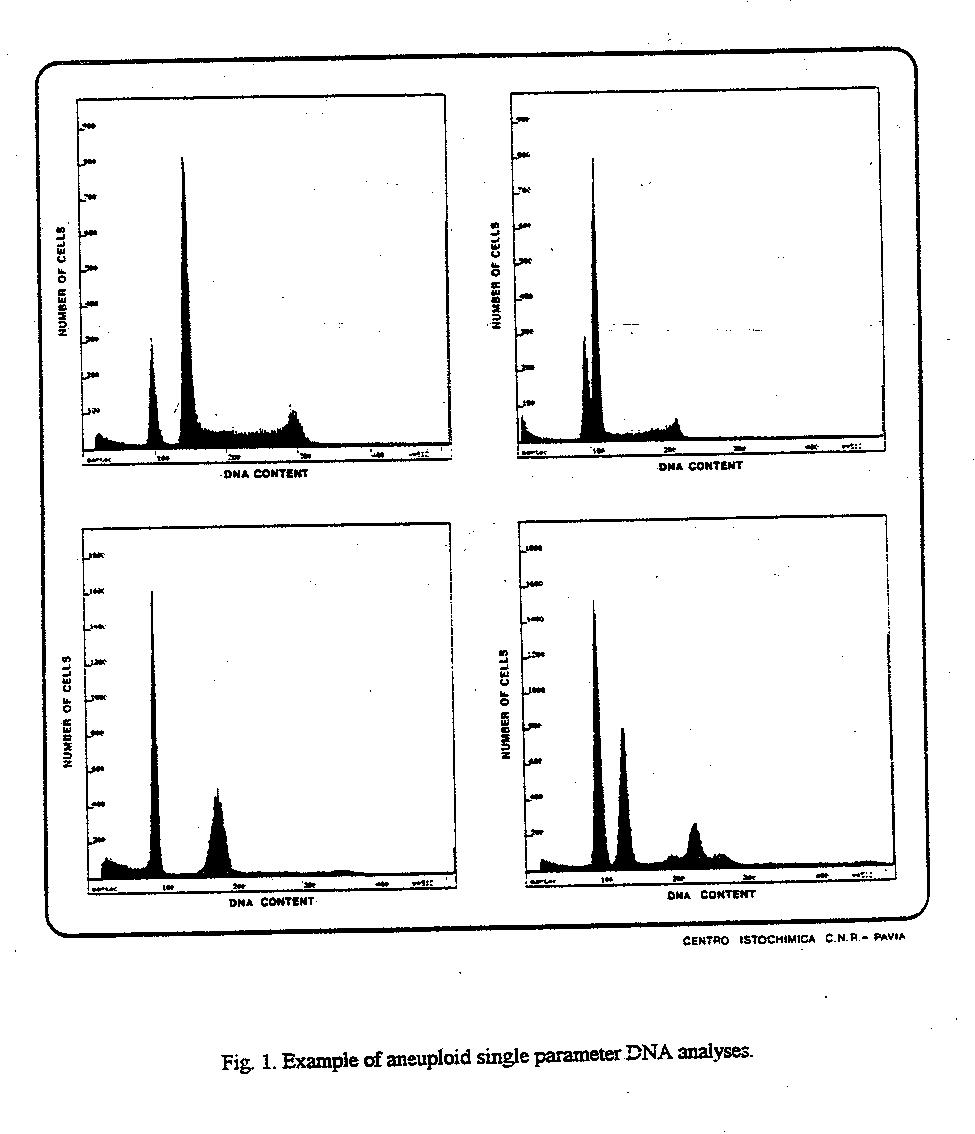
Fig. 1. Example of aneuploid single parameter DNA analyses.
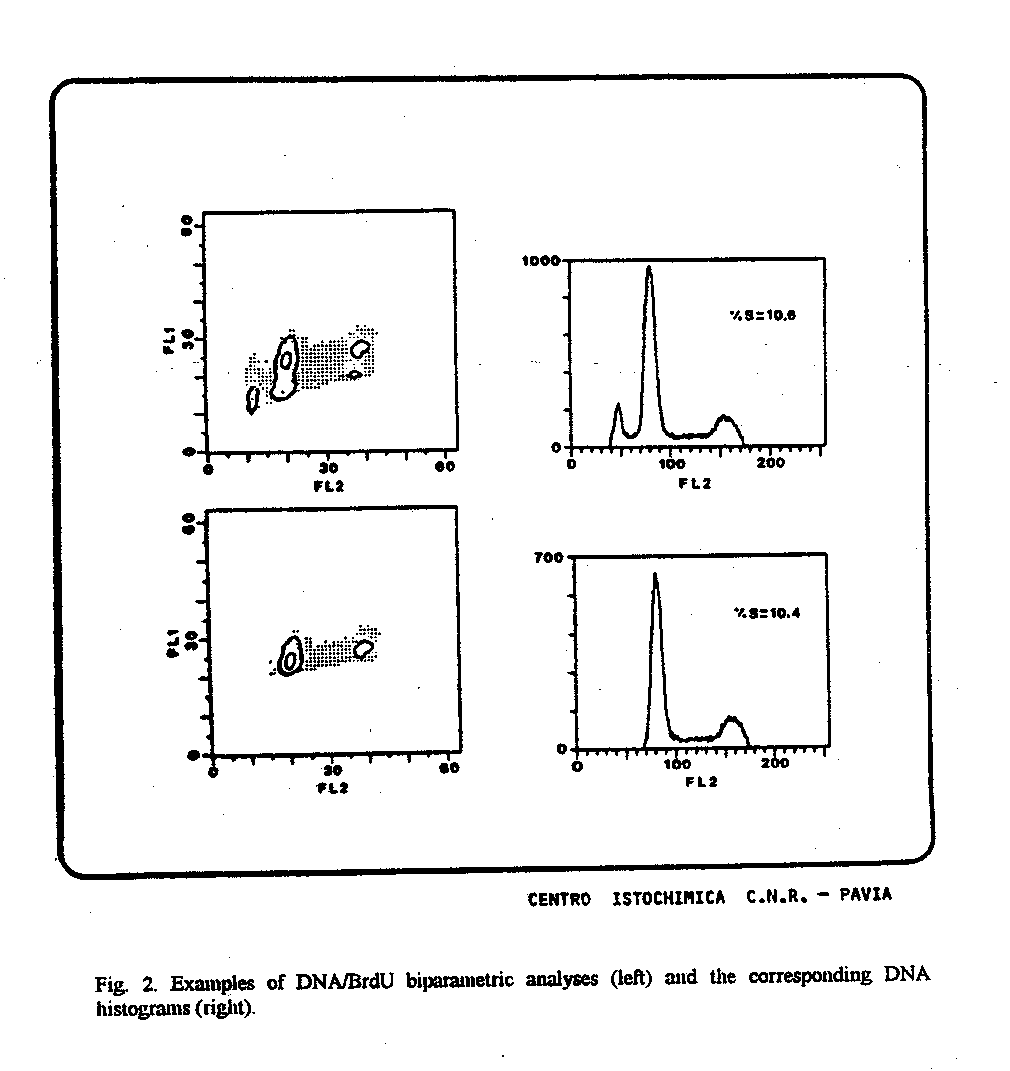
Fig. 2. Examples of DNA/BrdU biparametric analyses (left) and the corresponding DNA histograms (right).
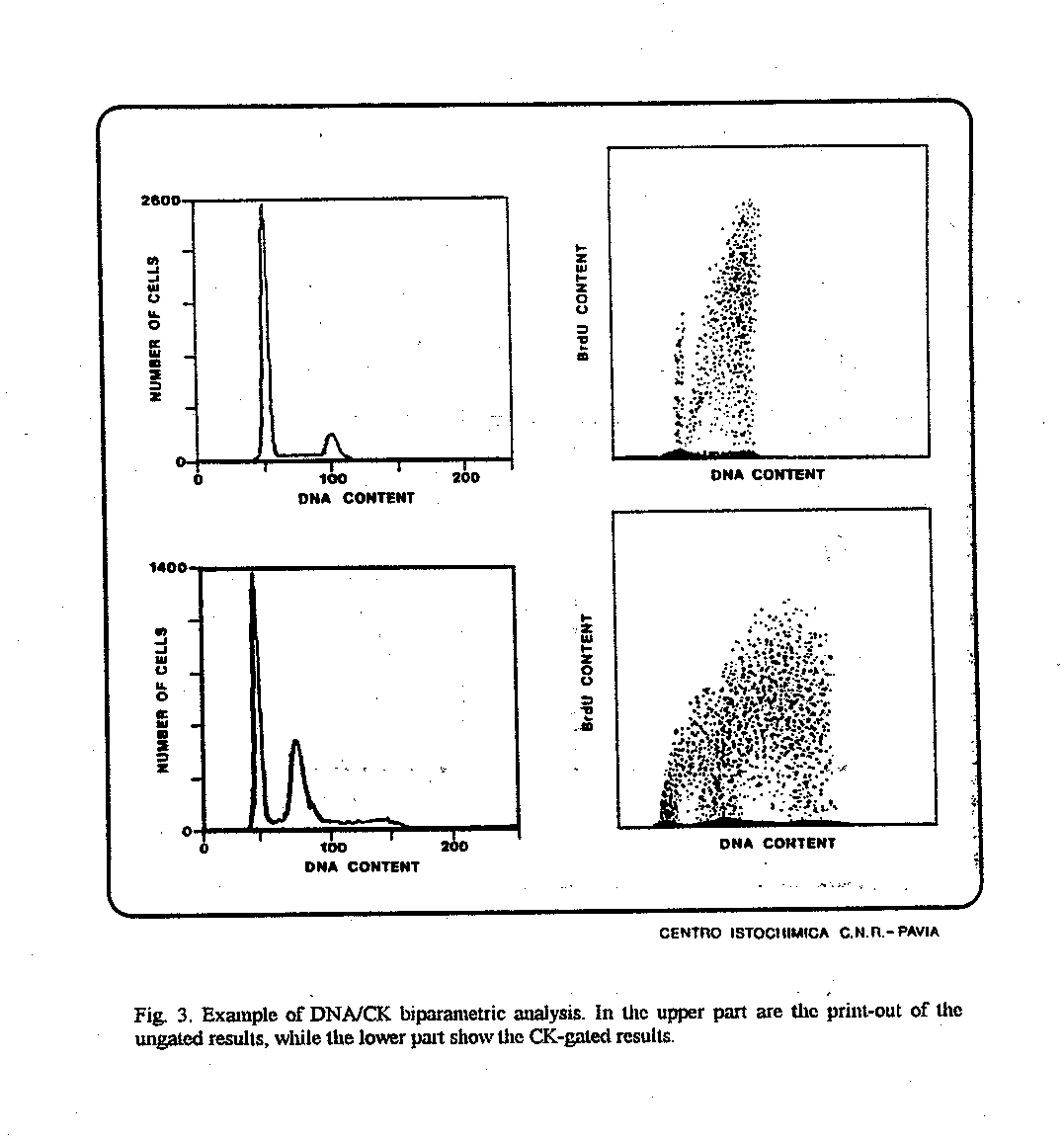
Fig. 3. Example of DNA/CK biparametric analysis. In the upper part are the print-out of the ungated results, while the lower part show the CK-gated results.
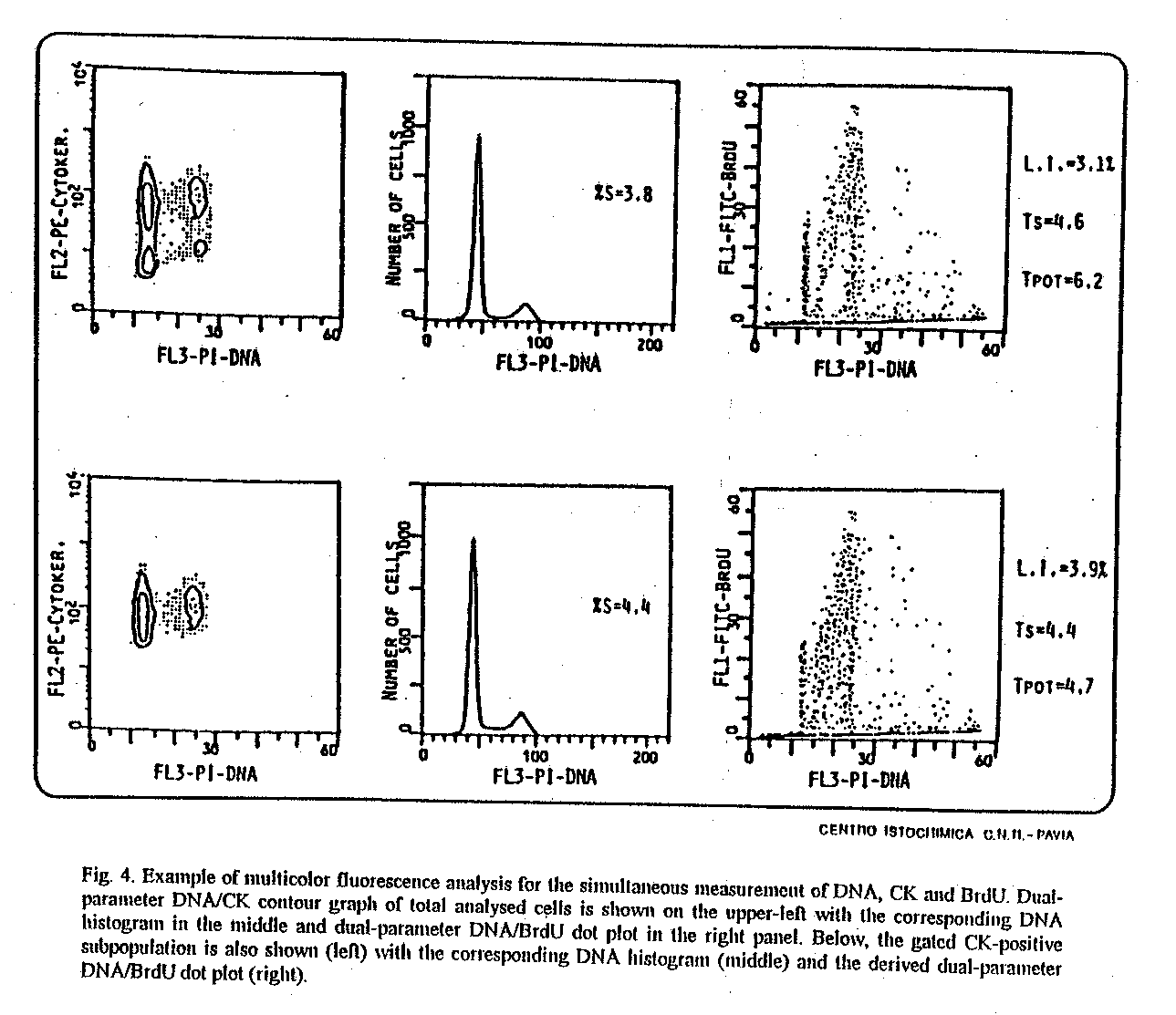
Fig. 4. Example of multicolor fluorescence analysis for
the simultaneous measurement of DNA, CK and BrdU. Dual-parameter DNA/CK
contour graph of total analysed cells is shown on the upper-left with the
corresponding DNA histogram in the middle and dual-parameter DNA/BrdU dot
plot in the right panel. Below, the gated CK-positive subpopulation is
also shown (left) with the corresponding DNA histogram (middle) and the
derived dual-parameter DNA/BrdU dot plot (right).
 |
 |
 |
 |
 |
CD-ROM Vol 3 was produced by Monica M. Shively and other staff at the Purdue University Cytometry Laboratories and distributed free of charge as an educational service to the cytometry community. If you have any comments please direct them to Dr. J. Paul Robinson, Professor & Director, PUCL, Purdue University, West Lafayette, IN 47907. Phone:(765) 494-0757; FAX (765) 494-0517; Web http://www.cyto.purdue.edu, EMAIL cdrom3@flowcyt.cyto.purdue.edu