

Hapten anti-hapten labeling systems for detecting weakly expressed antigens by flow cytometry.
Weakly expressed antigens on cells are often difficult to detect with fluorochrome-conjugated antibodies. Using secondary antibody or avidin-biotin systems can amplify the signal produced by a primary bound to a weakly expressed antigen, but these techniques are often plagued by high background fluorescence. One way to circumvent this problem is to label cells with hapten-conjugated primary antibodies (such as biotin, fluorescein, DNP, TNP, etc) followed by a secondary antibody directed against the hapten. This secondary antibody can be fluorochrome-coupled, or can be subsequently followed by a fluorochrome-labeled tertiary antibody against the species of the secondary anti-hapten. This system is surprisingly effective in many systems, both by its amplification of signal and by the low non-specific binding of many anti-hapten antibodies. For some systems biotin anti-biotin labeling can exceed the sensitivity of biotin-avidin labeling, with reductions in secondary reagent non-specific binding often seen with avidin and streptavidin. Hapten anti-hapten has traditionally been used in ELISA assays but is now being applied to flow cytometry as well. Anti-biotin, anti-fluorescein and other anti-hapten antibodies are widely available commercially (Sigma, Molecular Probes and Kirkegaard Perry Laboratories are good sources).
(Below). Examples of cell surface immunophenotyping. Tertiary labeling systems are complicated to set up but give the greatest signal amplification, often with low signal to noise ratios.
Below, data is shown for detecting CD30 ligand on T cells by labeling with a FITC-CD30 fusion protein, followed by an rabbit anti-fluorescein antibody and a FITC-anti-rabbit IgG (as in the bottom example of the above figure). This "triple sandwich" allows the amplified detection of CD30 ligand on cells with minimal increase in non-specific background and detects extremely weak CD30 ligand expression during T cell activation. Other fluorochromes (such as PE) can be used in place of FITC on the tertiary antibody. In such cases, other FITC-conjugated antibodies can still be used in conjunction with an initial FITC labeling of the weak antigen, since the anti-fluorescein antibodies quench their fluorescence.
(Below). Labeling procedure for detection of CD30 ligand expression using CD30 fusion protein and hapten-anti-hapten detection.
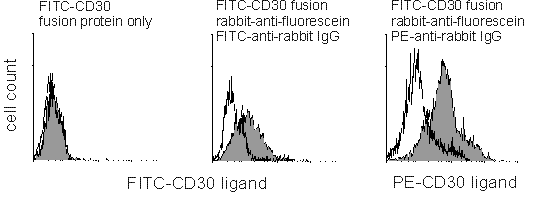
(Below). CD4-gated B6 mouse T splenocytes stimulated for 48 hours with crosslinked anti-CD3 antibody and IL-2 and phenotyped for CD30 ligand expression using either FITC-CD30 fusion protein only (left), fusion protein followed by anti-fluorescein and a tertiary FITC-anti-rabbit IgG (middle) or the same hapten-anti-hapten system using a PE-conjugated tertiary antibody (right). Unfilled and filled peaks are for isotype-matched control and CD30 fusion protein, respectively. The first two histograms were analyzed with the same detector voltage setting; note the negligible increase in background fluorescence in the hapten-anti-hapten sample.
![]() This
page is still being added to! More later!
This
page is still being added to! More later!
Back to the HSS Flow Cytometry Core Facility Home Page