

Detection of steroid-regulated plasma membrane Ca/Mg-ATPase (PMCA) activity in inside-out membrane vesicles by flow cytometry.
A collaboration with Dr. Michele Wheatly and Jennifer Weil in the Department of Biology, Wright State University.
This project explores the use of the flow cytometer to detect and measure calcium influx into isolated membrane vesicles. Such methodology has the potential for allowing the study of biochemical mechanisms not possible in intact cells, yet in a more physiological condition than as isolated proteins. It could also in some cases replace currently used radioactive methods for measuring ion pump activity in membrane vesicles.
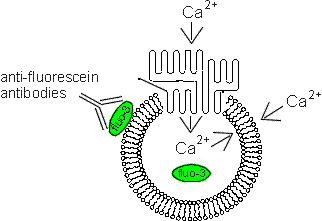
To use this technique, cells are lysed and allowed to reseal as vesicles in the inside-out orientation (so that PMCA will pump calcium ions into the vesicle interior). During the resealing process, the vesicles are loaded with the fluorescent calcium chelator fluo-3. The vesicles are then analyzed on the flow cytometer after addition of calcium and/or ATP, and the fluo-3 fluorescence measured. The amount of ATP-dependent calcium uptake is taken to be the level of PMCA activity in the vesicles. These studies were originally carried out in mouse T lymphocyte vesicles are are being expanded to other cell types, including crayfish cells, a model system for steroid-modulated PMCA activity.
Reference:
Telford, W.G. and Miller, R.A. Detection of plasma membrane Ca2+-ATPase activity in mouse T lymphocytes by flow cytometry using fluo-3-loaded vesicles. Cytometry 24:243-250 (1996).
(Below). A diagram illustrating the principle of PMCA activity detection of fluo-3-loaded vesicles. Anti-fluorescein antibodies are used to quench fluo-3 fluorescence originating from outside the vesicle. In principle, only calcium transported across the plasma membrane into the vesicle will result in a positive signal.
Back to the HSS Flow Cytometry Core Facility Home Page.