

Instrumentation.
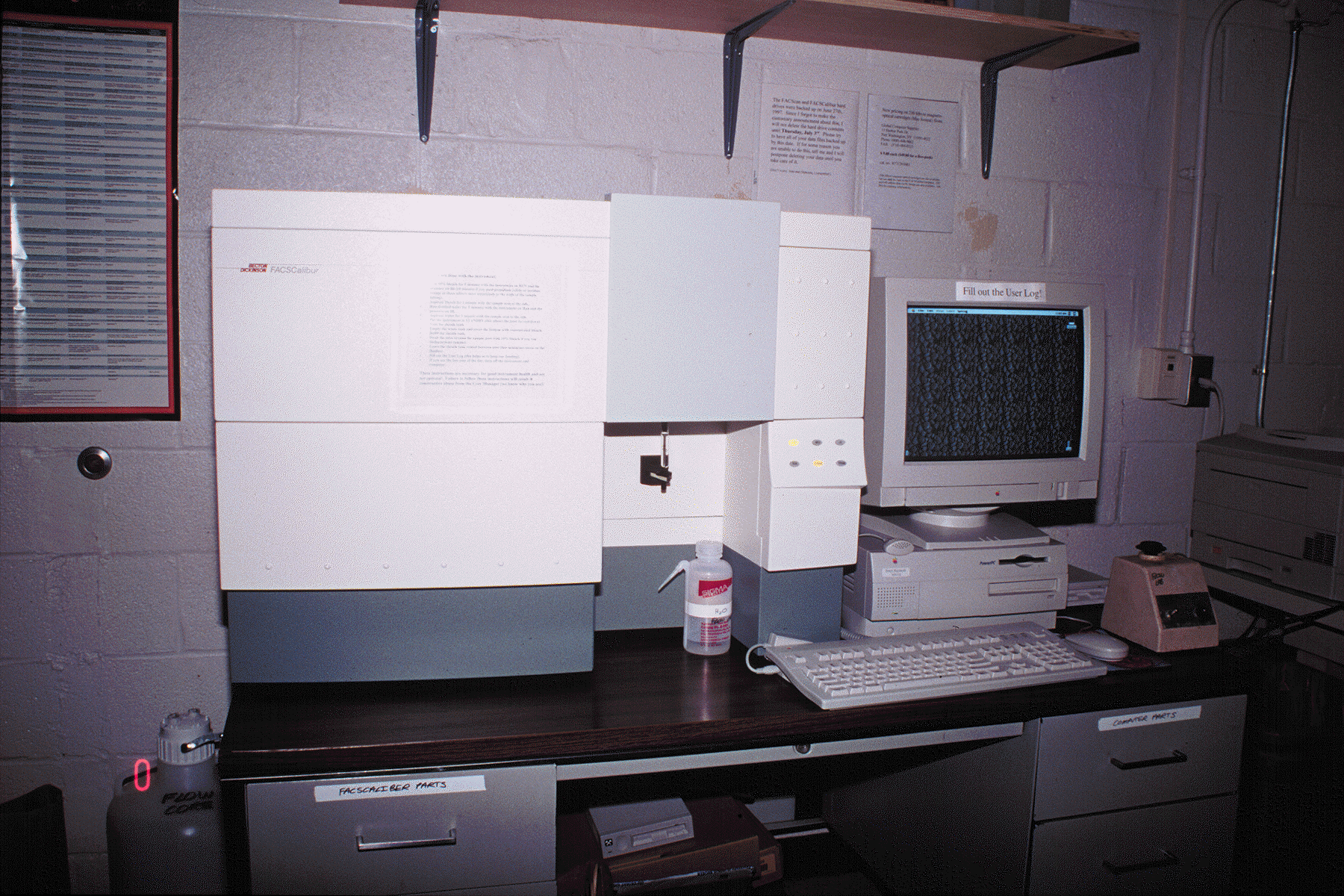
We currently have three flow cytometers available. Our Becton-Dickinson FACScan can analyze up to three fluorescent parameters simultaneously in addition to forward and side scatter using a single air-cooled argon laser, and has pulse processing capability for accurate cell sizing and DNA analysis. We also have a Becton-Dickinson FACSCalibur; this instrument can simultaneously analyze four fluorescent parameters using a dual argon and diode laser system. While the FACScan requires the use of fluorochromes that can be excited by an argon 488 nm laser line (such as fluorescein, phycoerythrin, propidium iodide, etc)., the FACSCalibur will also be able to take advantage of diode laser 630 nm laser-excited probes, such as allophycocyanin and Cy5. Both the FACScan and the FACSCalibur use Macintosh computers (Quadra 650s and PowerPCs) for data acquisition and analysis using the B-D CellQuest software package. All computers are equipped with 230 MB magneto-optical cartridge drives for data archiving.
The FACScan and FACSCalibur can be used by anyone who has received the appropriate training. If you have never used a B-D flow cytometer before, please contact Bill to set up an appointment for instrument instruction. Training on both instruments will take approximately two hours and is provided free to all users. Please do not attempt to use these instruments without training.
The FACSCalibur was purchased with a generous donation from The Fannie E. Rippel Foundation.
(Below). Our Becton-Dickinson FACScan.

(Below). Our Becton-Dickinson FACSCalibur.
Click here to see examples of cell analysis on the FACSCalibur.
We also have a Becton-Dickinson Vantage cell sorter. This instrument can detect up to five fluorescent parameters simultaneously. The instrument is normally configured with a Coherent Enterprise argon laser providing simultaneous excitation at 488 and 357 nm (in the UV), and a Spectra-Physics helium-neon laser emitting at 633 nm (in the red). In addition to the fluorochromes nornally used with the FACScan, the Vantage can take advantage of UV-excitable probes, such as the phenotyping fluorochromes Cascade Blue and AMCA, the DNA probes Hoechst 33258 and DAPI and the calcium indicator indo-1. We also have a removable Coherent Innova 90 krypton ion laser that can provide more intense UV and red laser excitation. The combined flexibility of the laser and filter configuration makes possible an almost limitless number of experimental designs with a variety of fluorochromes. In addition, the Vantage can sort cells (under sterile conditions, if desired) at rates up to 2500/second, either into tubes or into 24- and 96-well plates in predetermined numbers using an automatic cell deposition unit. These features makes the Vantage ideal for selective cloning and cell purification prior to PCR amplification. Our instrument has been retrofitted with the B-D Macrosort system, which uses interchangeable nozzles ranging fro 50 to 400 um for sorting a wide variety of particle sizes. Our Vantage has also been retrofitted with an upgraded data acquisition system, temperature-controlled sample chamber and a Cytek Time Zero kinetic module (shown in the photo below) for kinetic measurements of cell physiological parameters (such as calcium flux). Use of the Vantage requires the assistance of a trained operator, as well as prior consulting time to design your experiment.
(Below). Our FACSVantage. The Cytek Time Zero module is sitting on top of the instrument. For a diagram and a more detailed description of the instrument's current laser and optical configuration, click here. Click here to see an example of 5-color analysis on the Vantage.

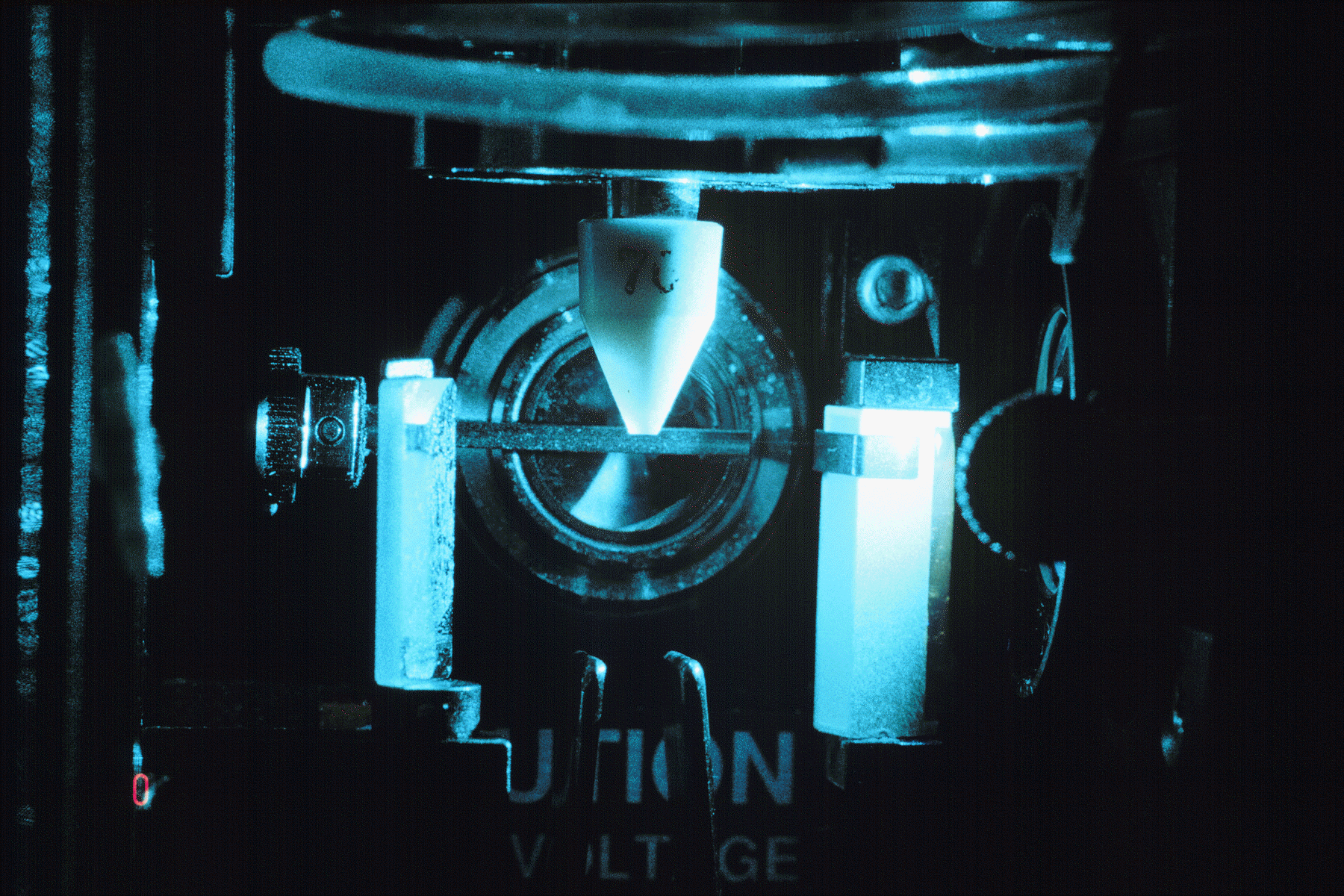
(Below). Detail of the FACSVantage nozzle assembly, shown here with a 70 um nozzle. The Macrosort function allows nozzle sizes from 50 to 400 um for sorting a wide variety of particle sizes.
(Below). The Vantage helium-neon laser, with steering optics in the foreground.

Users from HSS and outside institutions are charged a reasonable fee for instrument use. Click here for a fee schedule.
Back to the HSS Flow Cytometry Core Facility Home Page.